

It is the arrangement of electrons into shells and subshells that most concerns us here, so we will focus on that. Any s subshell can hold up to 2 electrons p, 6 d, 10 and f, 14. Different subshells hold a different maximum number of electrons.Thus, the first shell has only a single s subshell (called 1 s), the second shell has 2 s and 2 p subshells, the third shell has 3 s, 3 p, and 3 dand so forth. The subshells of each shell are labeled, in order, with the letters s, p, d, and f. The first shell has only one subshell, the second shell has two subshells, the third shell has three subshells, and so on. Shells are further divided into subsets of electrons called subshells.Shells do not have specific, fixed distances from the nucleus, but an electron in a higher-energy shell will spend more time farther from the nucleus than does an electron in a lower-energy shell. Electrons are organized according to their energies into sets called shells (labeled by the principle quantum number, n). Generally the higher the energy of a shell, the farther it is (on average) from the nucleus.We say that the energies of the electrons are quantized. Electrons in atoms can have only certain specific energies.
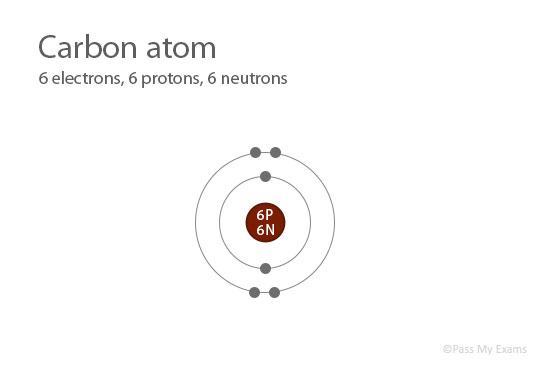
It makes the following statements about electrons in atoms: The modern theory of electron behavior is called quantum mechanics. Do they move around the nucleus at random, or do they exist in some ordered arrangement? To describe how electrons are grouped within atoms.Īlthough we have discussed the general arrangement of subatomic particles in atoms, we have said little about how electrons occupy the space about the nucleus.


 0 kommentar(er)
0 kommentar(er)
